Nikon D4 200mm macro w/close-up F32, 1/3" ISO 160 3R1 flashes with a little translucent packing material over them.
Thanks for any advice.



Moderators: rjlittlefield, ChrisR, Chris S., Pau



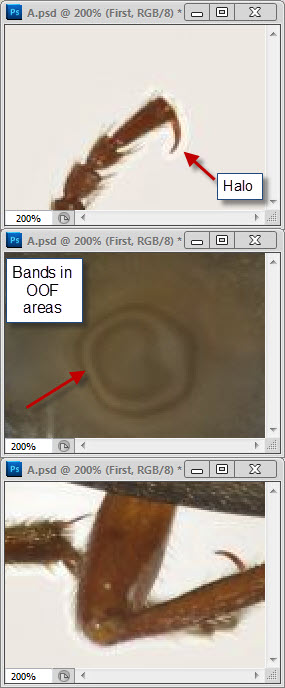
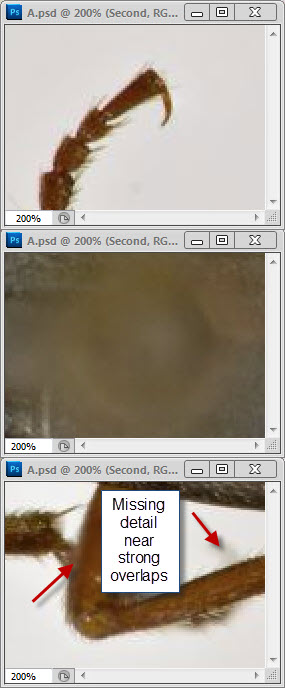
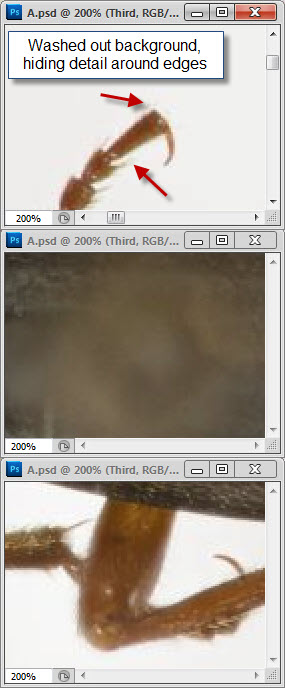
Well, your three posted images are three different sizes, all 1024 pixels wide but with heights of 761, 726, and 812 pixels. When I pull those images into Photoshop, make a layered stack of them, and use transparency to compare alignment, I see that the beetles are slightly different sizes and orientations. No two of them can be made to line up exactly, although the first and third are pretty close.robirdman wrote:I tried to make things as equal as possible.
The bands I pointed out are in what I assume is the head of the insect pin. Without knowing which software and what method you're using, I can't tell you what caused them. Bands that look like this often arise in depth map methods when there is significant difference in illumination from one frame to another. They can also be produced by out-of-order frames. Neither of those seems to be a good explanation for why they appear in your image, so I'm treating the origin as a mystery and I'm just saying "here's another thing to look out for when stacking".What is the oof area?
Beats me -- try it and see. In my own experience, the best results seem to come from letting the stacking software see things as they go continuously in and out of focus. Stacks with large gaps often have more obvious artifacts such as contamination halos around foreground features. But for your purposes the savings in time to shoot the stack may be a good tradeoff against whatever more artifacts you get.For the pin head, just add a extra focus point without all the ones in between?
Again, you'll have to decide what that means in terms of resolution and specimen prep and posing. I don't know anything about these beetles. I do know a bit about butterflies, and across the ones I know, "enough detail" can range anywhere from a fuzzy snapshot of the wing pattern down to obscure details of the genitalia that can be seen only by dissection and high magnification.I want to have enough detail for species ID or confirmation whenever possible.
That makes perfect sense. Helicon's Method B is a depth map method, similar to CombineZ's Do Stack and Zerene Stacker's DMap, but with no provision like Zerene's contrast threshold slider to designate where you care about smooth transitions versus sharp detail.robirdman wrote:the one with banding in oof area is Helicon but method B.

