You see perfect circles (or concentric circular isochromes) when you are looking straight down the optical axis of an uniaxial crystal. Gemologist use this method to find out whether a mineral is uniaxial/biaxial isotropic/nonisotropic. You are also seeing in the circle a black center spot, called the melatope, and a cross centered in the melatope and the cross is called the isogyre.
My experience is that you get these circles quite often, but I don't know what determines whether you get circles or something else. There are quite a lot of varibales at play. The difference between water and acetone/methanol is the speed of evaporation. With acetone crystallization takes place very fast while it takes hours for the water to evaporate from the slide. Melting introduces heat and many minerals have different crystal structures depending on the temperature during crystallization. Available space is also affecting the growth of crystals and you can manipulate this aspect by using very thin layers of the saturated solution and also by tilting the slide during crystallization so that you force the solution to spread as thinly as possible. Or you could make a small pile of paracetamol on the slide and keep on dropping acetone on the pile repeatedly. This would result in a thicker crystals.
When you are forcing the crystallization to take place under a cover slip or between slides, you are forcing the crystallization to take place "2-dimensionally". This is probably also affecting the crystallization very much, and maybe also result in crystal growth perpendicular to your natural axis of view. Then you would be looking "automatically" down the optical axis of the crystals... maybe...
So I would say that the main factors at play here are heat, speed of crystallization and spatial freedom to grow. The interference colors are also affected very much by the thinness/thickness of the crystals. I don't have any litterature to offer, gemology or petrology textbooks could be a place to search.
I have images of many different crystal forms. Might create another post to display this incredible variation, even with a single substance like paracetamol.
If you are using semicrossed polarizers the colors vanish, as compared to crossed polarizers. To reintroduce new and different colors, one needs to try out various wave plate retarders of various thicknesses. I have found out that the clear cellophan wrap used by florestry shops to wrap around the flowers is quite qood for this purpose. One can cut pieces from this wrap and make thicker retarders by attaching these on top of each other. I have a number of these ready made and try out different thicknesses and different amounts of crossing the polarizers. Addicting stuff as the shapes and colors just keep on transforming.
Hope you don't mind me posting an image here. This is just to give an idea of using cellophan wraps as wave plate retarders
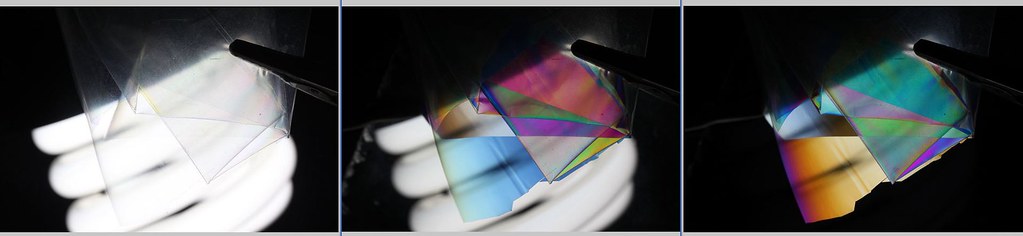 Photographing light: DIY wave plate retarder
Photographing light: DIY wave plate retarder by
Henri Koskinen, on Flickr




