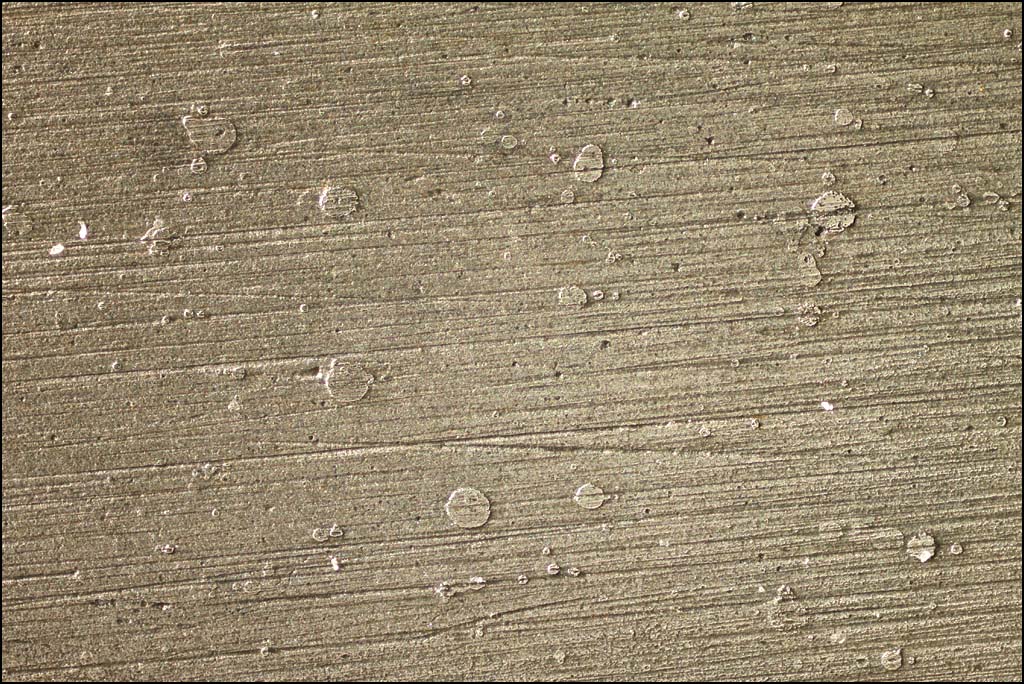
A wider view, 4X on sensor (5.5 mm field width), previous area at lower left:

And wider yet (38 mm field width), with different lighting:

The story here is that I left some stainless steel forceps sitting in a bath of diluted Lime Away for a day or so. It was an accident to do that, but if I had thought about it, I probably would have said "No problem, those weak organic acids won't do anything to stainless steel anyway."
Hah, wrong on both counts!
When I noticed the forceps again, the portions under liquid looked almost black, obviously quite affected. I pulled them out, rinsed them off, and casually rubbed with steel wool, expecting the surface to come clean.
Wrong again!
The surface did clean up quite a bit, but its finish was obviously very different on the soaked and unsoaked portions. Of course I couldn't resist looking under a microscope.
What you see above is roughly what I saw through the scope. The portions under the liquid were actually etched quite significantly. Aside from the change in general luster, there are now a lot of small circular "islands" of steel that rise above the general level. Such raised spots did not appear before, so I can only presume that they represent places where the steel did not get etched away. I'm thinking that perhaps those were places where a bubble formed on the metal and kept the acid solution from circulating.
The third picture shows what the forceps look like under room light. The etched portion, on the left, appears dark and matte, while the original surface is bright and mirrorlike. The second picture, on the other hand, show what the surface looks like when illuminated from the side. The mirror-like portions go dark because they don't reflect into the lens, leaving the matte portions to become relatively much lighter. The same lighting in the first picture just shows the raised dots more clearly.
I hope you find this as interesting as I did!
--Rik
Technical: Canon T1i camera and electronic flash. Image #1 is with a Nikon CFI BE 10X objective on Mitutoyo MT-1 tube lens, stacked at 10 microns; image #2 is with Nikon CFI BE 4X, also on the MT-1, stacked at 25 microns; image #3 is with Canon 100mm f/2.8 L USM macro lens, single frame at f/11. These are uncropped and with no CA correction, just the way they came out of the camera and stacking.
