Abbe's formula for resolution = 1.22λ/(NAobj+NAcond)
My setup is as follows:
0.55 NA LWD condenser
EC Plan-Neofluar 10x/0.3 Ph1
EC Plan-Neofluar 20x/0.5 DIC
EC Plan-Neofluar 40x/1.3 oil Ph3 DIC
Plan-Neofluar 63x/1.25 oil Ph3
Plan-Apochromat 63x/1.4 oil DIC
Plan-Apochromat 100x/1.4 oil DIC
The images were acquired with a Hamamatsu ORCA-AG monochrome camera with CCD size 1344x1024. I used a green interference filter (roughly 550nm) for these tests and set up critical illumination for each objective. I will not go into details for the lower magnification objectives, but you can see the results in the first overview image.
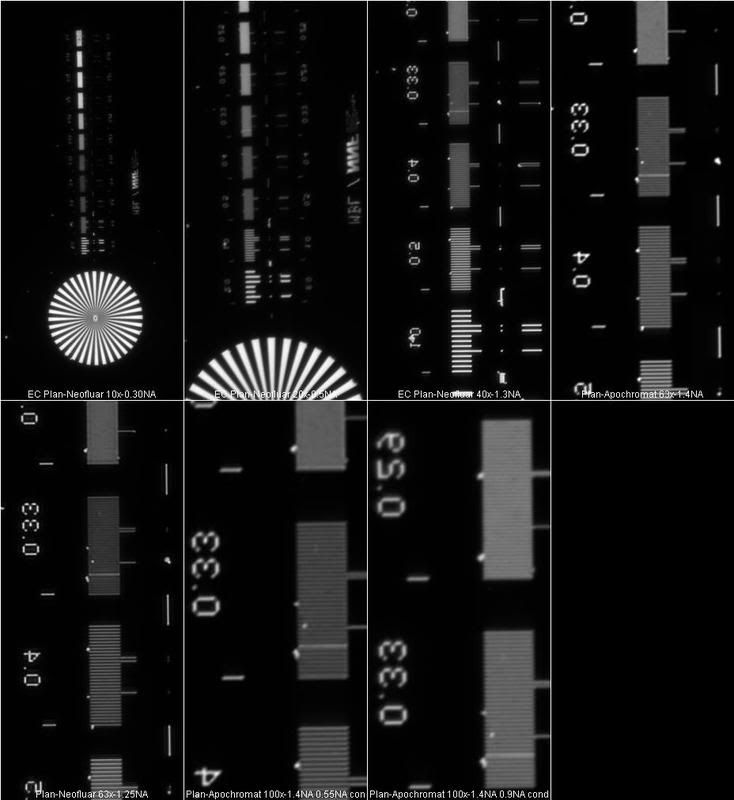
For 40x and above, I took a closer look at the contrast difference in the rulings. According to the Rayleigh criterion, an object is resolved if the contrast difference between points is 24% or greater.
The 40x 1.3NA EC Plan-Neofluar objective is a bit much for this camera and ideally, I should have 1254 vertical pixels to capture all of the resolution, so I am undersampling a tad. Nevertheless, I will include the results, but not the analysis...
Theoretical resolution:
(1.22*550nm)/(1.3+0.55)= 363nm
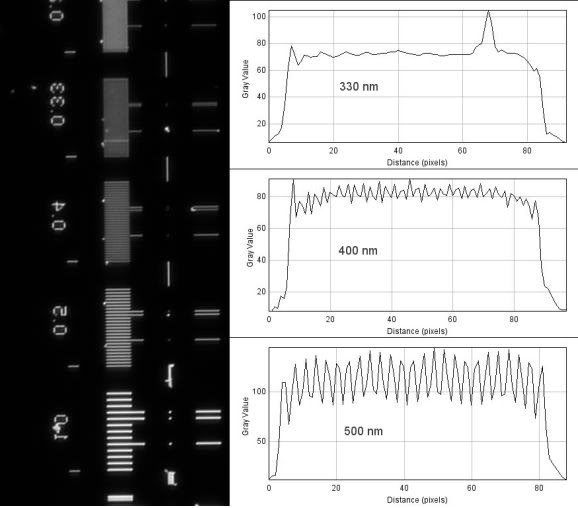
The 63x 1.4NA Plan-Apochromat...
Theoretical resolution:
(1.22*550nm)/(1.4+0.55)= 344nm
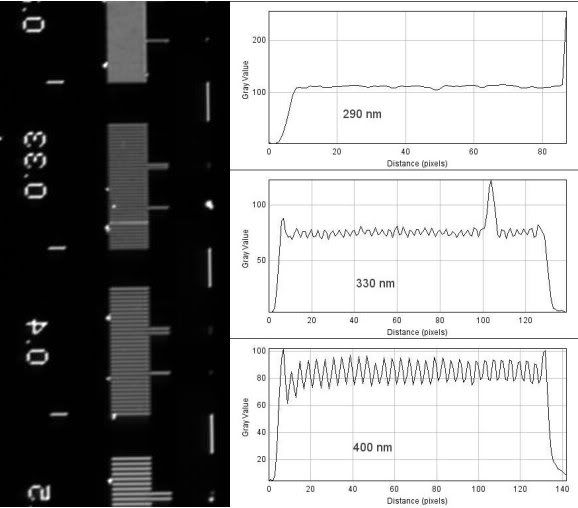
The 100x 1.4NA Plan-Apochromat...
Theoretical resolution:
(1.22*550nm)/(1.4+0.55)= 344nm
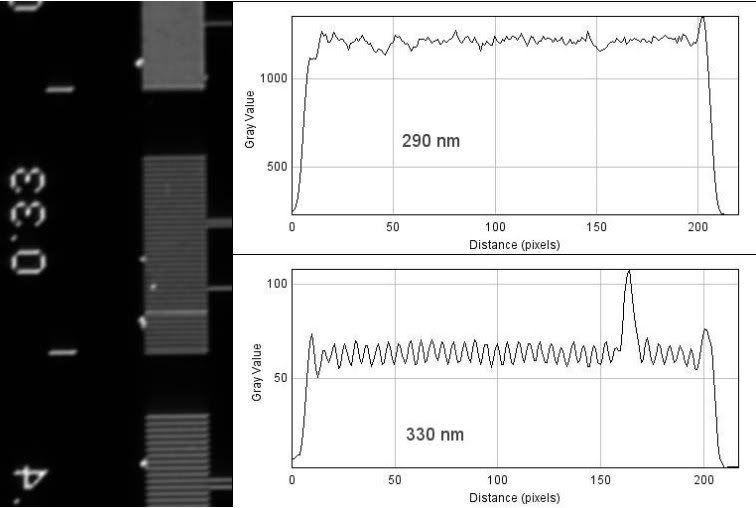
And finally, I used a 0.9NA condenser in place of the 0.55NA LWD to see what I might gain. Again, with the 100x 1.4NA Plan-Apochromat...
Theoretical resolution:
(1.22*550nm)/(1.4+0.9)= 292nm
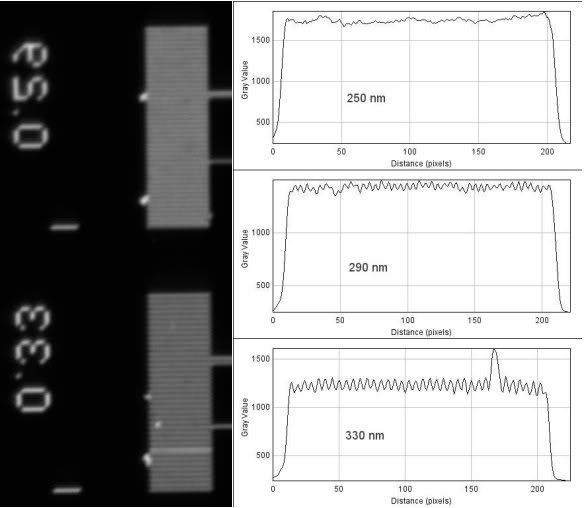
As you might be able to deduce from the line plots, seeing does not necessarily mean resolving according to the standard definition. With the 1.4NA objectives, I can resolve objects that are closer than 400nm, and although you can clearly see the rulings of 330nm, they are not fully resolved (only around 9-10% drop in intensity). With the 0.9NA condenser, you being to see clear periodicity in the 290nm rulings, but again, they are not fully resolved - I think part of this is due to a slightly out of focus image.
I have also taken images with the Nikon D70 DSLR as well, but I have not yet analyzed the results. It will be interesting to see how the interplay of a larger chip size and the RGB Bayer pattern influence the results. I will post back here when I've done this.
In the future, I hope to track down a 1.4NA condenser as well as a monochromator so I can demonstrate the influence of wavelength.
Chris