Shucks, I've written that myself.
I thought you might be interested in a direct illustration of that idea.
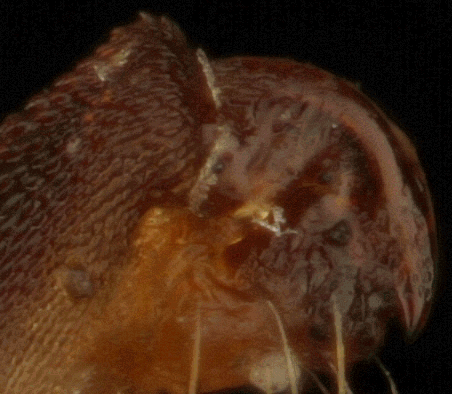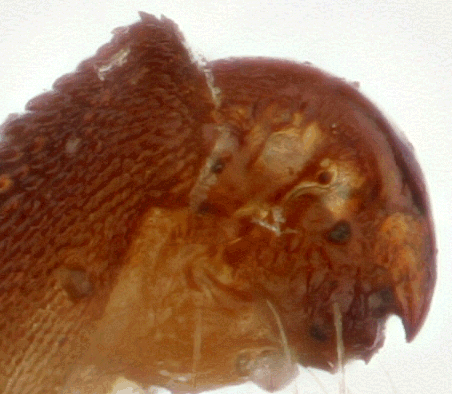
What we have here are three pictures of the tail end of a yucca moth, two shot with an SEM at the lab where I work, and one as an optical stack on my kitchen table, using a macro lens on bellows.
Background and technical information is given here. The only new snippet is that this particular stacked image was created this evening using TuFuse Pro 0.9.3, with no manual retouching.
In the stacked image, you can see a bit of the common "show through" visibility error, in this case where background bristles appear to be visible behind a semi-transparent foreground structure, when in fact the foreground structure is completely opaque and the background bristles were actually seen by a wide-aperture lens looking around the foreground structure while focused on the background.
--Rik